Exelixis spikes on positive Phase 3 study of cancer drug
 The shares of genomics-based biopharmaceutical company Exelixis (EXEL) recorded a new 12-month high of $7.34 yesterday. The reason for the 11.23% gain in the share price is the positive result in the Phase 3 trial of kidney cancer drug Cabometyx.
The shares of genomics-based biopharmaceutical company Exelixis (EXEL) recorded a new 12-month high of $7.34 yesterday. The reason for the 11.23% gain in the share price is the positive result in the Phase 3 trial of kidney cancer drug Cabometyx.
However, as explained below, the uptrend should be used as an opportunity to exit from the long position in the counter.
In April 25th , 2016, the San Francisco-based company’s drug Cabometyx was given a nod by the US FDA (Food and Drug Administration) for the treatment of patients suffering from advanced Renal Cell Carcinoma (RCC). The patients were earlier treated by anti-angiogenic therapy. Clinically meaningful improvements were seen during the phase 3 trial for the patients suffering from the advanced kidney cancer. All three efficacy parameters (Overall survival, Progression-Free Survival, and objective response rate) showed considerable progress. Based on the results, the FDA awarded Fast Track and Breakthrough designations to Cabometyx.
Exelixis, along with its partner Ipsen, announced the positive results of Cabometyx. The drug company also stated that it would announce the positive Phase 3 trial results at the annual meeting of the American Society of Clinical Oncology (ASCO), which runs June 3rd to 7th in Chicago.
Regarding the drug, Bernard Escudier, chair, Genitourinary Oncology Committee, Institut Gustave Roussy, stated that
CABOMETYX demonstrated a clinically meaningful benefit for those with bone metastases, which is encouraging for physicians and patients who are seeking additional therapeutic options.
Michael M. Morrissey, President and chief executive officer of Exelixis said that
We are dedicated to exploring the full potential of CABOMETYX to help as many patients as possible.
According to the FDA, Phase 3 trial normally continues for a time period of 30.5 months. Once there are satisfactory results, the company should apply for the final approval (‘New Drug Application’) from the FDA. The time frame for approval would be six months to two years. Thus, considering the time period involved in the process, we can say that it will take several years for the commercialization of the drug. Any uptrend based on the news related to the trials is normally short lived. So, fundamentally, we can expect the price to revert back to its original level, once the excitement over the announcement fizzles out.
Technically, the stock has completed a perfect rounding bottom pattern. In such circumstances, as shown in the image, the target price is calculated by adding the depth of the pattern to the breakout level. So, going with that theory, we can expect the price to reach about 8.20. The RSI reading also indicates an overbought situation.
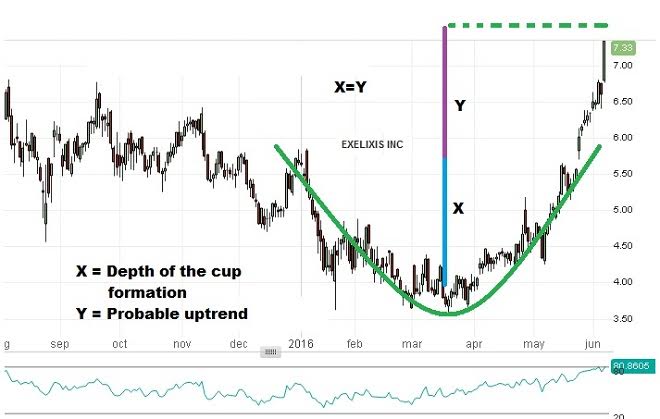
Thus, near the 8.20 levels, a one touch put option trade should be initiated by a binary trader. To ensure higher chances of success, an expiry date in the first week of July should be chosen. The suggested target price for the contract is $6.50 or higher.
